Enter the Ground State Condensed Electron Configuration for F
The selenide ion has the same electron configuration as or is iso-electronic to KryptonThere are 8 electrons in its 4th shell 4th energy level. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2.

Electron Configuration For Fluorine F
Expert Answer 100 8 ratings Previous question Next question.
. Its electron configuration will be F. The f orbital can hold 14 electrons. How to do condensed electron configuration.
What is the energy that results from motion. For example He 2s2 2p2 should be entered as He 2s22p2. First find the required element on the periodic table.
Since it says Ni2 the 2 plus charge means that this is a Ni cation that has lost 2 electrons. The Noble gas electron configuration or the condensed electron configuration for F is He 2s2 3p5. Click to see full answer.
1s22s22p5 Now the F anion is formed when 1 electron is added to a neutral fluorine atom. Now find the atomic number of the first noble gas in the periodic table. Enter the ground-state condensed electron configuration for Ge.
Enter the ground-state condensed electron configuration for F. Ground state electron configuration of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. In this case the valence electrons of molybdenum are six.
Atoms can jump from one orbital to another orbital by excited state. In the bromine ground-state electron configuration five electrons of the 4p orbital are located in the 4p x 2 4p y 2 and 4p z sub-orbitals. 2p5 and the term symbol is 2P32.
In this regard what is the electron configuration for F. The p-orbital has three sub-orbitals. Which is the electron configuration for an atom of fluorine.
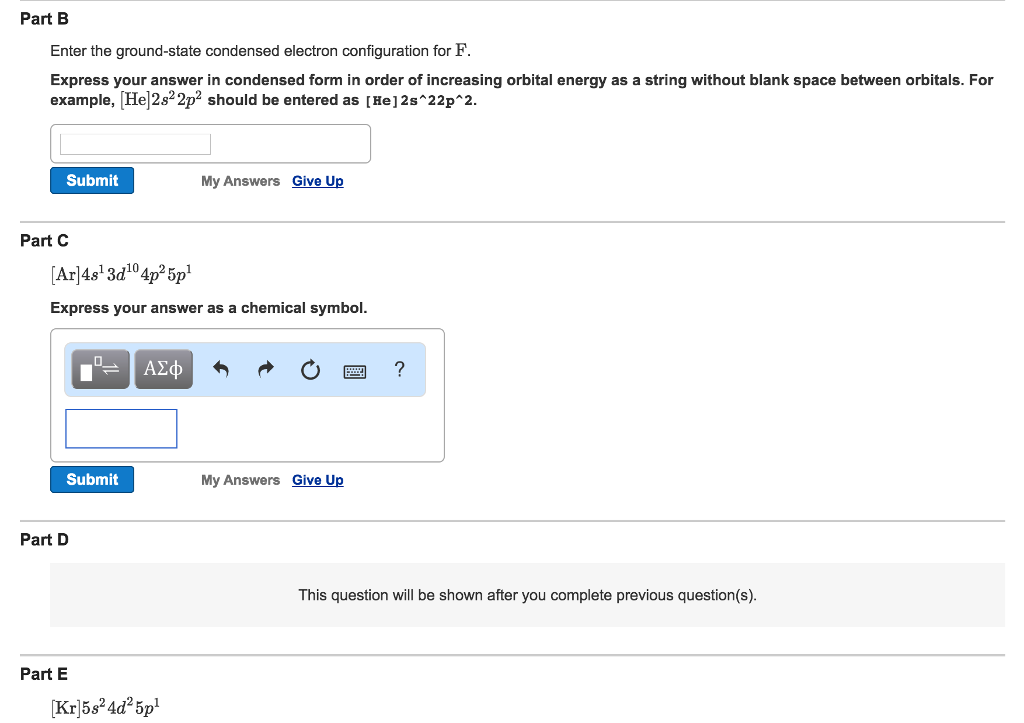
Ar 4s1 3d10 4p2 5p1 Express your answer as a chemical symbol. The p orbital can hold 6. This is called quantum jump.
For the Cr 2 ion we remove one electron from 4s1 and one from the 3d5 leaving us with. This method uses the previous noble gas and does not obey Hunds Rule - only a starting place. For example calcium is element 20.
Enter the ground-state condensed electron configuration for F. Molybdenum ionMo 3 electron configuration. These 2 electrons are lost from the electron orbital with the highest energy which in this case is the 4s orbital.
Oganesson element 118 is a good example to show the order of the orbitals. Ground state electron configuration of molybdenumMo is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 5 5s 1. The remaining five electrons will go in the 2p orbital.
The ground state electron configuration of ground state gaseous neutral fluorine is He. The condensed electron configuration of bromine element 35 is. The electron configuration of a neutral titanium atom is.
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p. The noble Gás notice for the configuration of Bromo trons is air. The configuration is arkan followed by 3.
Express your answer in condensed form in order of increasing orbital energy as a string without blank space between orbitals. Learn how to write electron configuration in condensed form. But the orbitals overlap.
The Madelung rule gives the order. The 4d subshell in the ground state of atomic xenon contains _____ electrons. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital.
The electronic ground state configuration is one is to to a school two P three. Without the 2 electrons in the 4s orbital the electron configuration of Ni is now Ar3d8 since the 4s orbital is empty. The two electrons that are lost when the Ti2 is formed will come from the 4s orbital which means that the electron configuration of the cation is Ti21s 2 2s 2 2p 6 3s 2 3p 6 3d 2.
Xe6 s 24 f 5. Also how many electrons are in. Thus the correct configuration is actually.
10 Type in the atomic symbol for the ground state element that matches. For the Cr 3 ion we remove a total of three electrons one from the 4s1 and two from the 3d5 leaving us with. Enter the ground-state condensed electron configuration for Nb.
The electron that we would have liked to believe is in the 5f orbital is actually placed in the 6d orbital. Identify the element and enter its ground-state condensed electron configuration. This electron configuration shows that the last shell of molybdenum has an electron and the d-orbital has a total of five electrons.
Express your answer in condensed form in order of increasing orbital energy as a string without blank space between orbitals. This give us the correct configuration of. Its electron configuration is.
This tells you that the neutral fluorine atom has a total of 9 electrons surrounding its nucleus. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Express your answer in condensed form in order of increasing orbital energy.
For example He2s22p2 should be entered as He2s22p2. Colorbluebarulstackrel Rn 5f3 6d1 7s2 Even though the 6d orbitals are higher in. Write the noble gas symbol in parentheses to start its electronic configuration.
What is the ground-state electron configuration of the fluoride ion F. Put the atomic number of the noble gas under this symbol so that you know the number of electrons. Fluorine is located in period 2 group 17 of the periodic table and has an atomic number of 9.
For example He2s22p2 should be entered as He2s22p2. Part C Which element has the following configuration. Enter the ground-state condensed electron configuration for F.
Enter the chemical symbol for the element. The following electron configurations represent excited states. Condensed ground state electron configuration for bromine.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 4. The d orbital can hold 10. The element here is nitrogen and the condensed electronic configuration will be helium followed by to s to to be three.
Solved Enter The Ground State Condensed Electron Chegg Com

No comments for "Enter the Ground State Condensed Electron Configuration for F"
Post a Comment